Current Projects
Project 1
Project 1
Project 1
Diet and exercise modulate the sperm epigenome in men
Project 2
Project 1
Project 1
Epigenetic information flow from
soma to male germline
Project 3
Project 1
Project 3
Transmission of epimutations from father to offspring
Project 1

Summary
This project will test our hypotheses that overweight and inactive lifestyle result in epimutations in
the sperm epigenome relative to the normal epigenetic programming in lean and active men and that diet and exercise modulation leads to reversal of these epimutations resulting in both a healthier “phenotype” and “epigenotype” which may persist after stopping the interventions.
Project Leader
Dr. Ronald S. Swerdloff
Dr. Christina C. Wang
Co-Investigator
Dr. Harry Rossiter
Dr. Rachelle Bross
Dr. Yanhe Lue
Significance
This study is timely and important because prevalence of obesity and inactivity may result in metabolic disturbances of the offspring through the sperm epigenome propagating a vicious cycle. Healthy eating and regular exercise may reverse sperm epimutations to a normal regulation of gene expression and disrupt the vicious cycle of the concept of “unhealthy” fathers, “unhealthy” epigenome and “unhealthy” children.
Project 2

Summary
This project aims to address one fundamental question that remains unanswered: given that the effects of exposures, either environmental or dietary, are presumably initially manifested as epigenetic changes indirectly exposed somatic cells (e.g. pancreatic islet cells, adipocytes, hepatocytes, etc.), how do the phenotype-specific epimutations in somatic cells get transduced into spermatozoa? Data to be obtained will help fill the knowledge gap in our understanding of the molecular mechanisms underlying the intergenerational epigenetic inheritance of paternally acquired traits in general.
Project Leader
Dr. Wei Yan
Co-Investigator
Dr. Qi Chen
Significance
The proposed study is of high significance because of the following: 1) It will uncover the hidden pathways for epigenetic information to flow from somatic cells to sperm and thus, become heritable. Data to be obtained will fill a significant knowledge gap in our understanding of the molecular mechanism underlying the intergenerational epigenetic inheritance of paternally acquired traits in general. 2) Transmission of the environment- or diet-induced epimutations from somatic to germ cells potentially represents a critical– and possibly manipulatable– step in the process of inter-/transgenerational epigenetic inheritance of disease phenotypes. Thus, the findings of this study may lead to preventive and/or therapeutic measures to stop disease transmission from fathers to offspring. 3) It has the potential to reveal, at least partially, the etiology of the global obesity pandemic, which is the leading cause of the soaring incidence of metabolic diseases.
Project 3
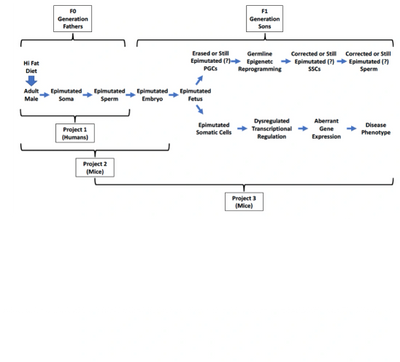
Summary
It is focused on investigating mechanisms underlying the sequential series of events that includes transmission of epimutations from the HFD/-Ex F0 sperm to F1 fetus to F1 immature pup to F1 adult to induction of epigenetic disease in F1 offspring. With its overall comprehensive scope, as well as its potential to very productively integrate with Projects 1 & 2, Project 3 will represent an in-depth study, the comprehensive nature of which is unparalleled in the field.
Project Leader
Dr. John R. McCarrey
Co-Investigator
Dr. Keren Cheng
Significance
The research proposed here is significant because it is designed to 1)identify the specific combination of epigenetic parameters involved in transmission of HFD/-Ex-induced epimutations from sperm to the ensuing fetus, 2) reveal the extent to, and mechanisms by, which resulting epimutations in the F1 fetus are propagated to developing somatic and germ cell lineages and on into the immature and adult offspring, 3) determine the extent of intercellular homo- versus hetero-geneity of HFD/-Ex-induced epimutations in spermatogenic cells of F0 sires and in relevant germ and somatic cell types in their F1 offspring, 4) discern the mechanisms by which inherited epimutations contribute to dysregulated gene expression in tissues relevant to aberrant phenotypes in the offspring, 5)elucidate dysregulated pathways responsible for defective or disease states among offspring of HFD/-Ex males, and 6)determine the extent to which the incidence of all of these deleterious effects can be reduced by transition of HFD/-Ex males to a normal (healthy) diet + exercise (ND/+Ex).
Copyright © 2022 National Center for Reproductive Epigenomics - All Rights Reserved.
Powered by GoDaddy Website Builder
